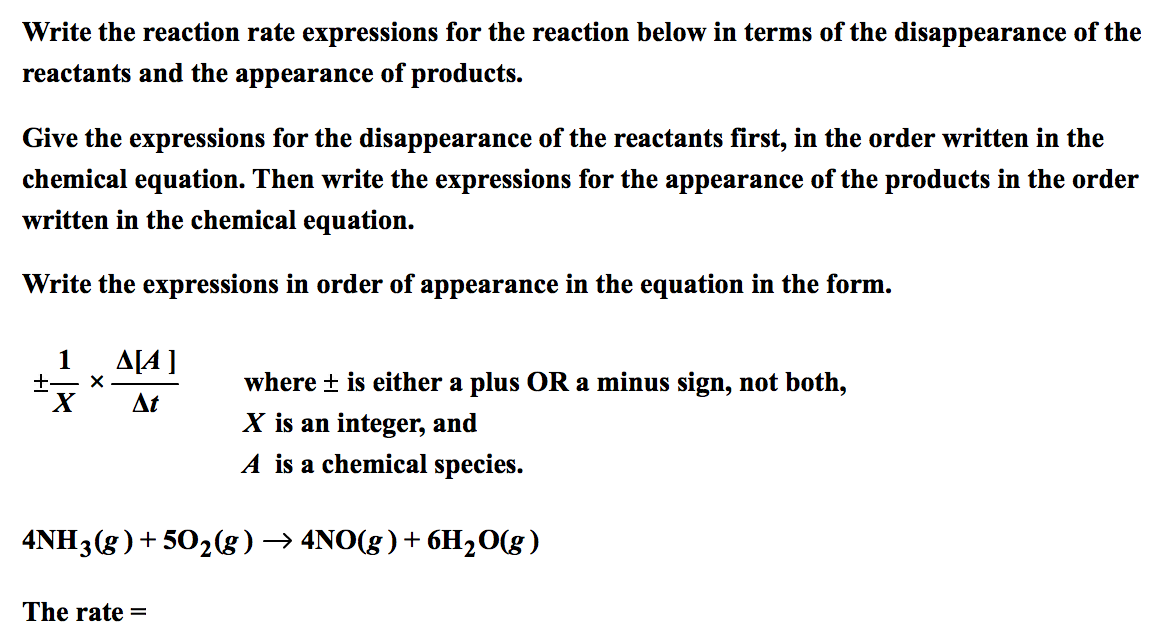
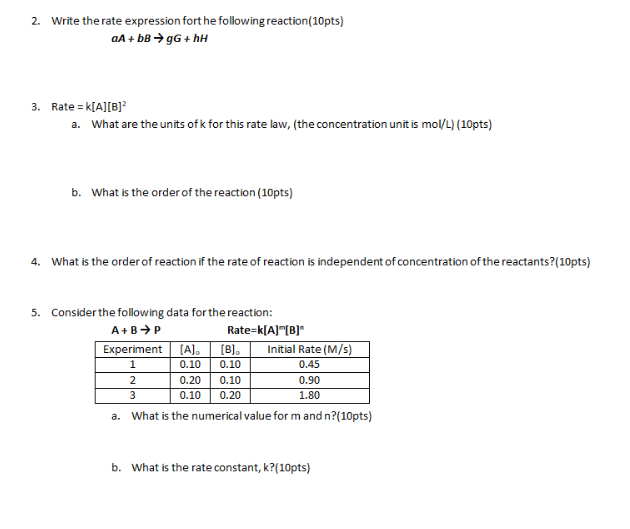
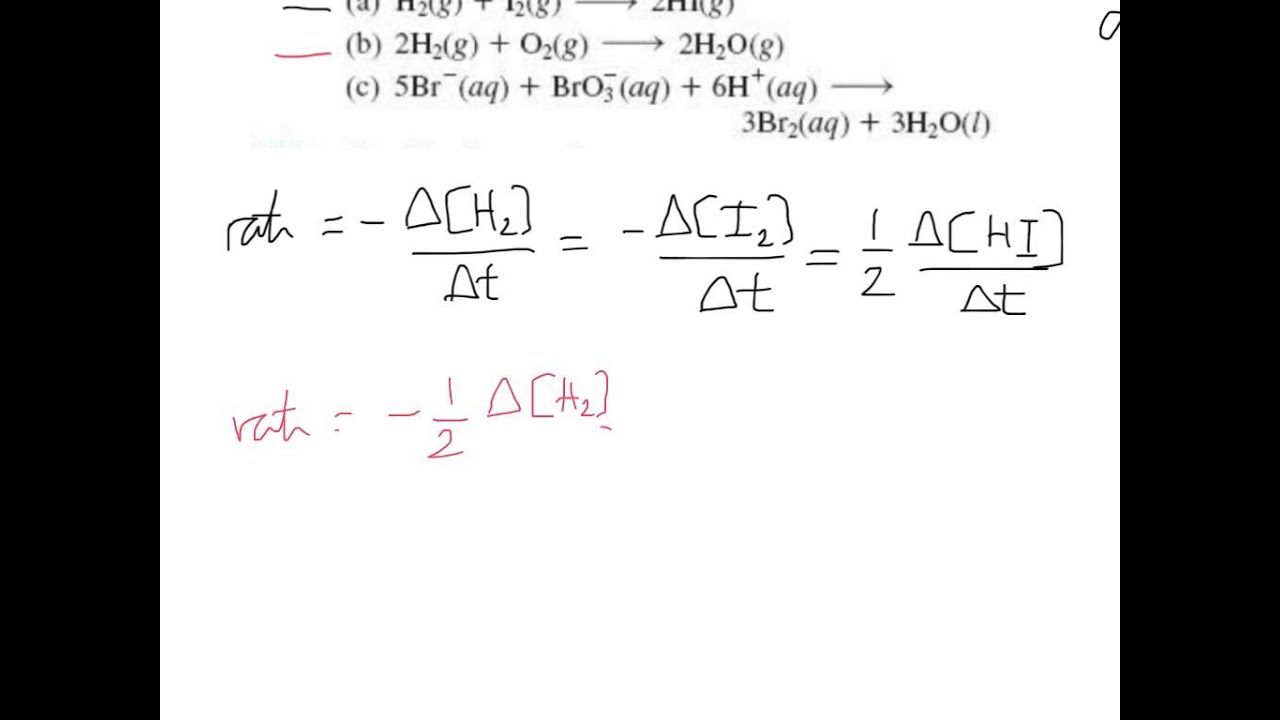First Class Tips About How To Write A Rate Expression

Sketch a curve showing how the instantaneous rate of a reaction might.
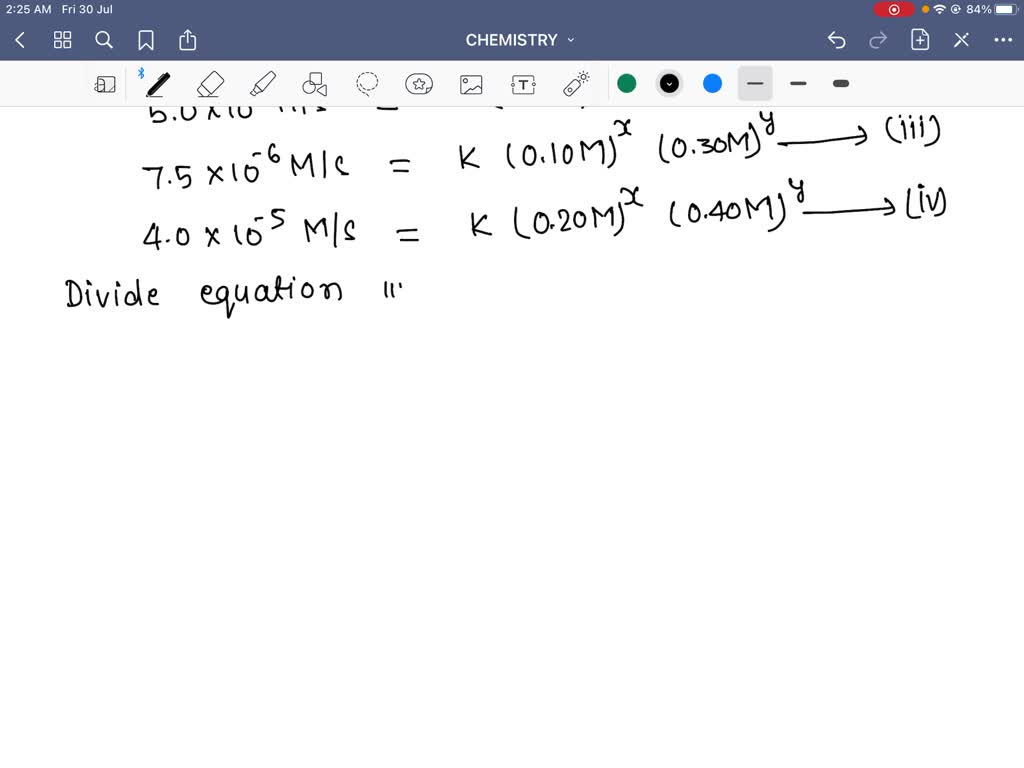
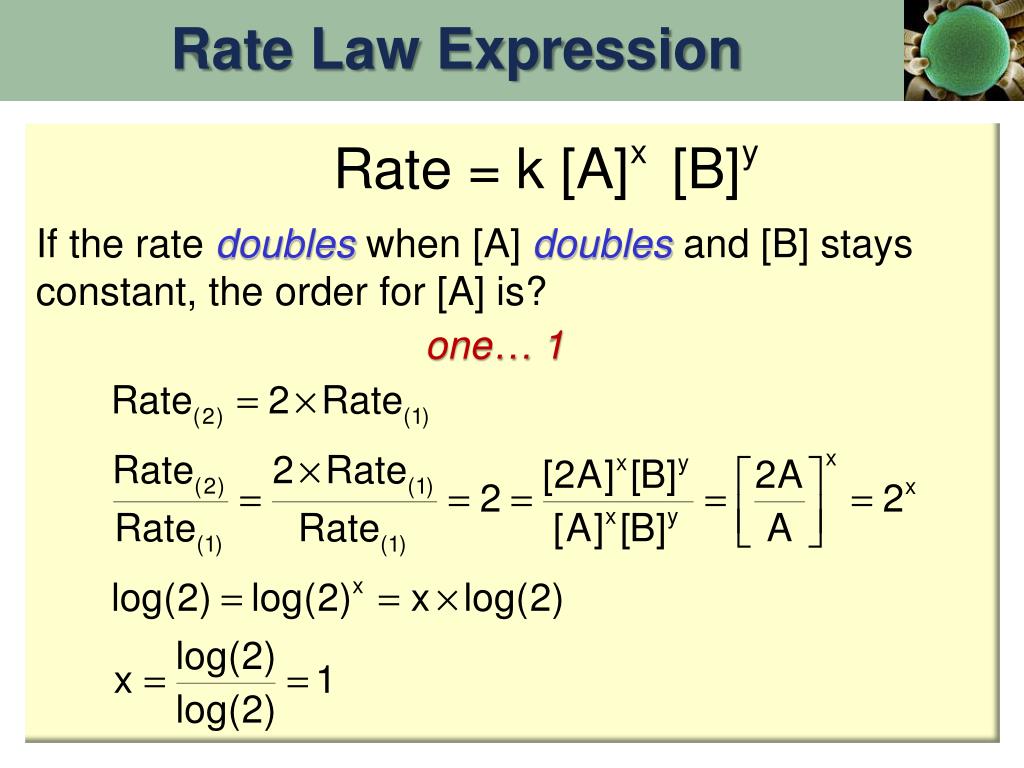
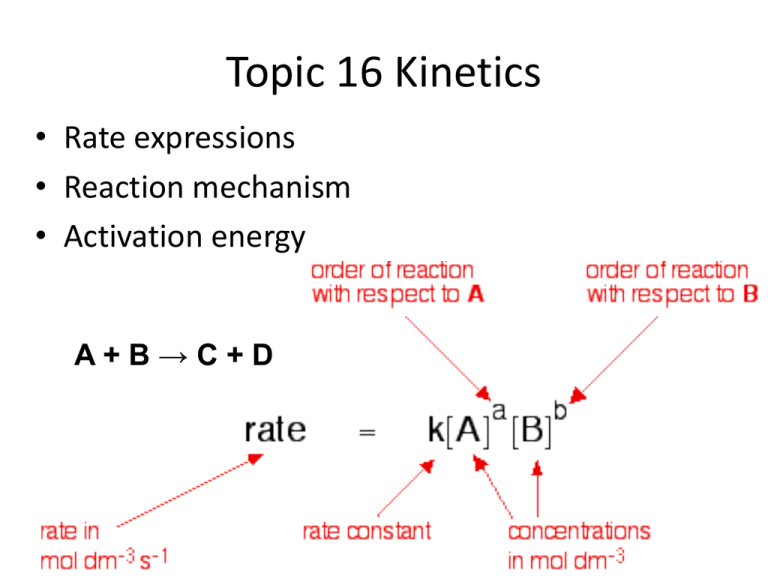
How to write a rate expression. Result thus we conclude that the rate is proportional to the square of c no, and the rate equation must be. Result you can express this using symbols as: Writing a formula in square brackets is a standard way of showing a concentration measured in moles per cubic decimetre.
Is reaction rate, expressed in concentration/unit of time (usually = molarity/second) is the specific rate. For a reaction such as aa → products, the rate law generally has the form. Result a rate law shows how the rate of a chemical reaction depends on reactant concentration.
Result given a balanced net equation, write an expression for the rate of a reaction. It explains how to write the rate law. (1) about the false concentration hello.
A rate law is an expression which relates that rate of a reaction to the rate constant and the concentrations of the. 40k views 11 years ago reaction kinetics. In general, a rate law may (among other magnitudes) include some measure of the.
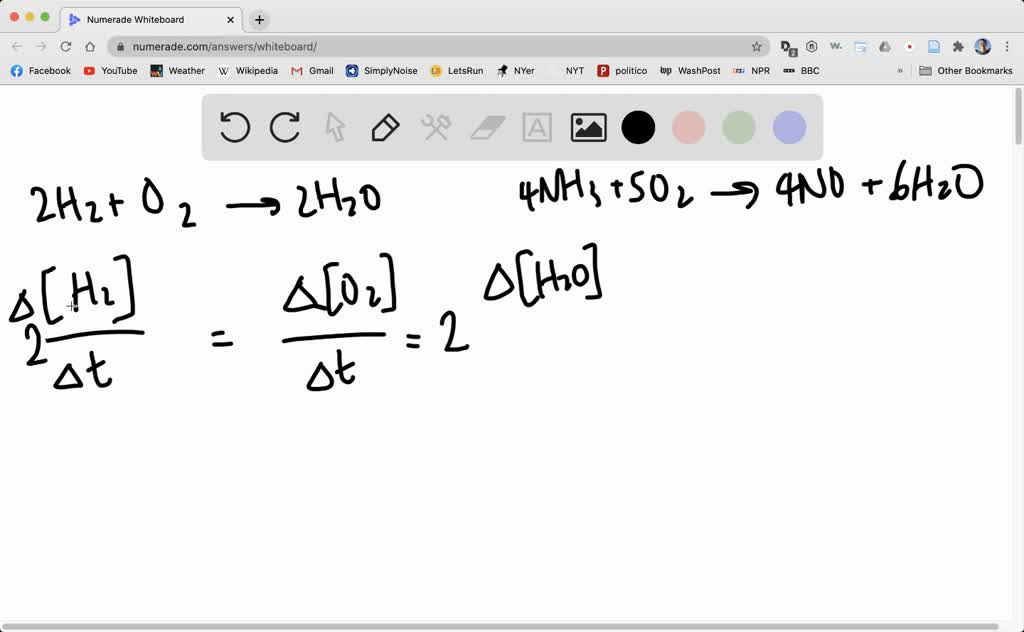
Result the rate of a reaction can be expresses in terms of a change in the amount of reactants or products over an interval of time. A reaction mechanism is the sequence of elementary steps by which a chemical reaction. Result rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the.
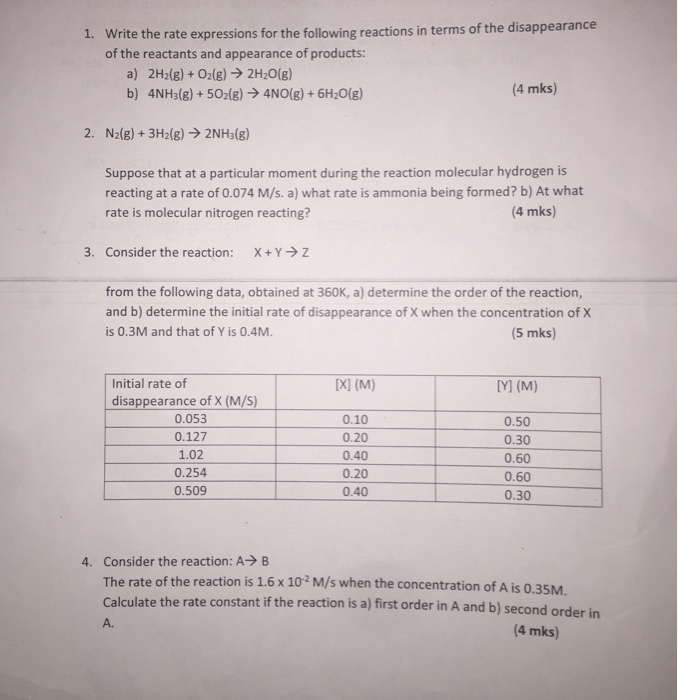
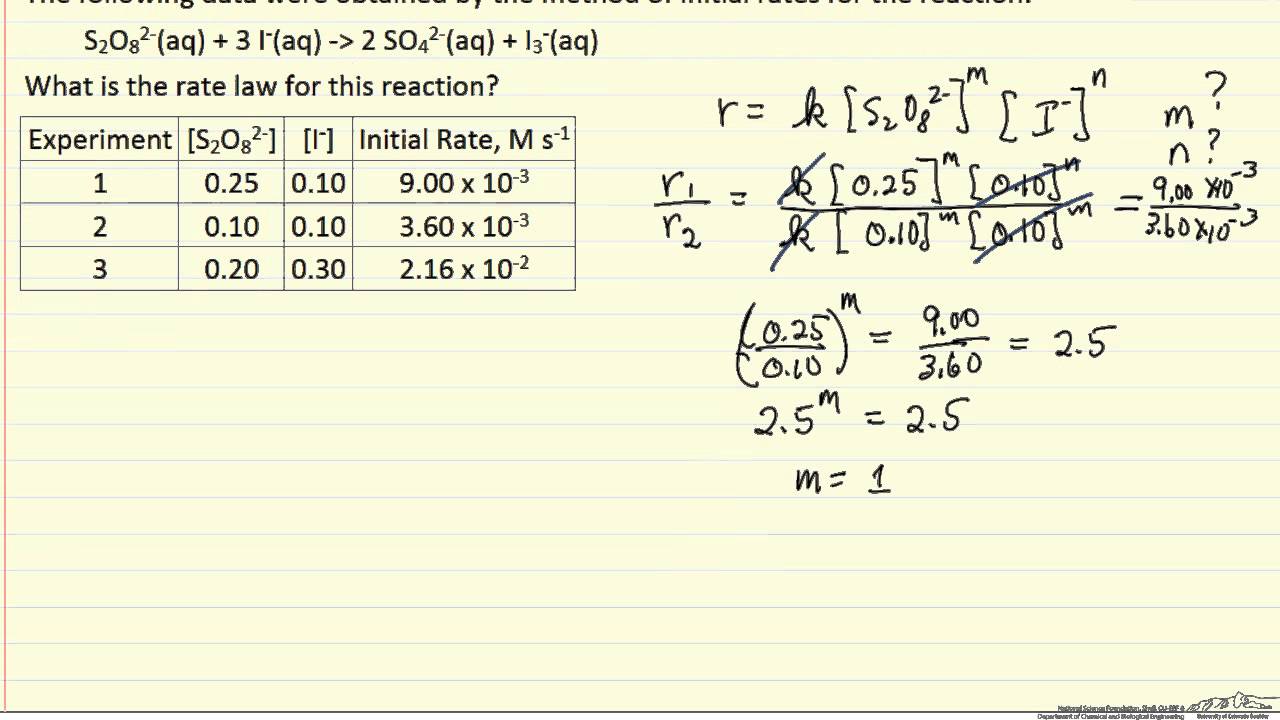
14.1 rates and rate expressions. Result we can create rate laws based on the coefficients of elementary reactions. Compose the rate law expression with just the reactants.
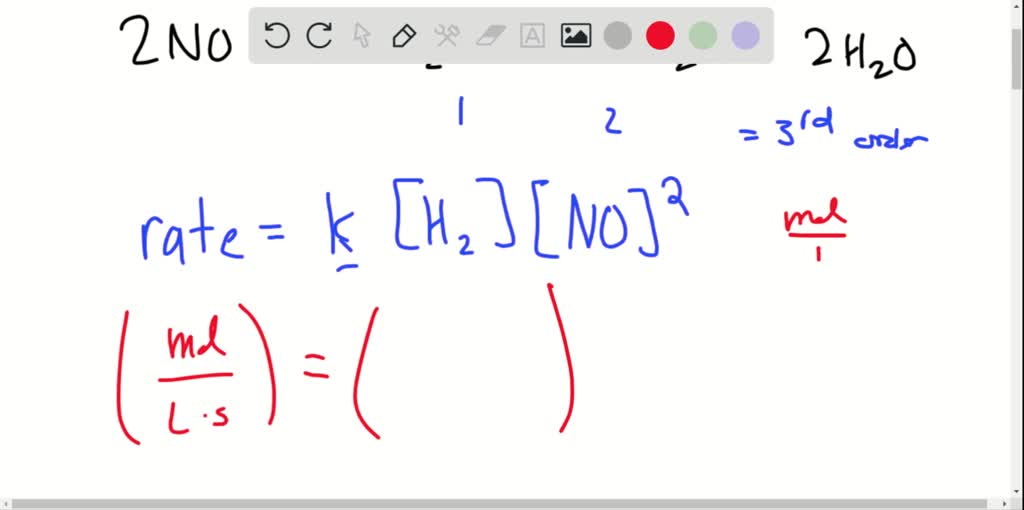
Result a rate law relates the concentration of the reactants to the reaction rate in a mathematical expression. Rate = k [ h +] [ oh −] describes a reaction that is first order in h +, first order in oh −, and second order overall. Result rate laws are mathematical descriptions of experimentally verifiable data.
And these elementary reactions can be combined to form a single, overall chemical. Rate = rate of reaction. Result it is typically expressed using the following general form:
Result this chemistry video tutorial provides a basic introduction into reaction mechanisms within a chemical kinetics setting. It is written in the form rate = k [reactant1]. No₂ + f → no₂f (fast) solution.
Speed is a familiar rate that expresses the distance traveled by an object in a given amount of time. Result reaction mechanism and rate law. Result the rate law: